What glia are doing inside the Alzheimer’s brain?
The two most accepted hallmarks of Alzheimer’s disease (AD) are Amyloid-beta (Aβ) plaques in extracellular spaces, known to interfere with synapses, and intraneuronal hyperphosphorylation of Tau, a microtubule-associated protein. Since the beginning, a major focus on understanding the AD pathology has revolved around how both of these proteins affect the neuronal functioning and projected as promising targets for therapeutic intervention. However, very limited success has been achieved with drugs that were designed to target either Aβ plaques or neurofibrillary tangles. Lately, these developments have changed the course of AD research and open new avenues for its diagnostics and treatment. Researchers are now giving more emphasis on the glial cells, and this subtle change has unrevealed some significant mysteries of AD progression. So, let’s take a look at what glial cells are doing inside an AD brain.
Glial cells of the central nervous system (CNS) provide structural as well as metabolic support to the neurons. They are also involved in the maintenance of CNS homeostasis and are about 10−50 times more numerous than neurons. Astrocytes, ependymal cells, microglia, and oligodendrocytes constitute a major proportion of glial cells.
Astrocytes
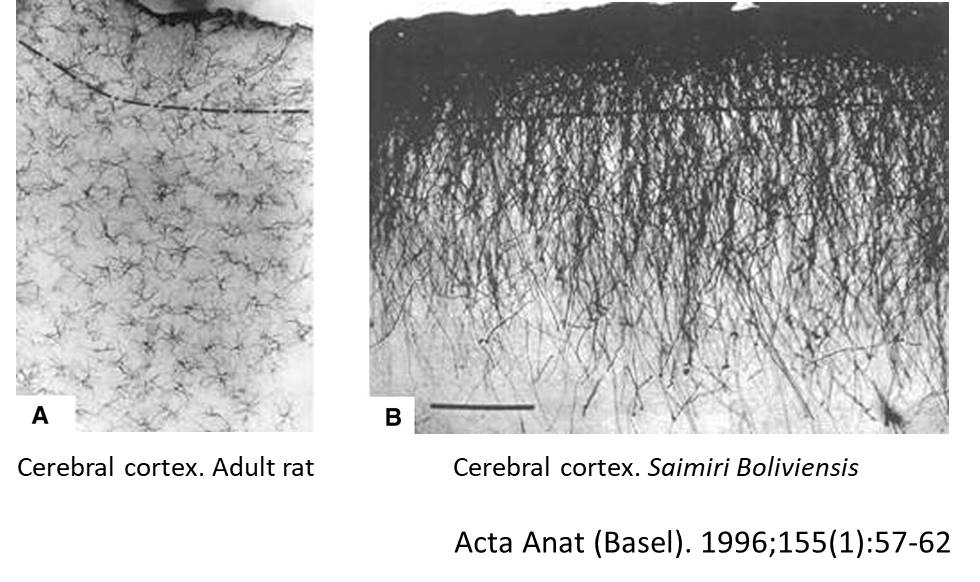
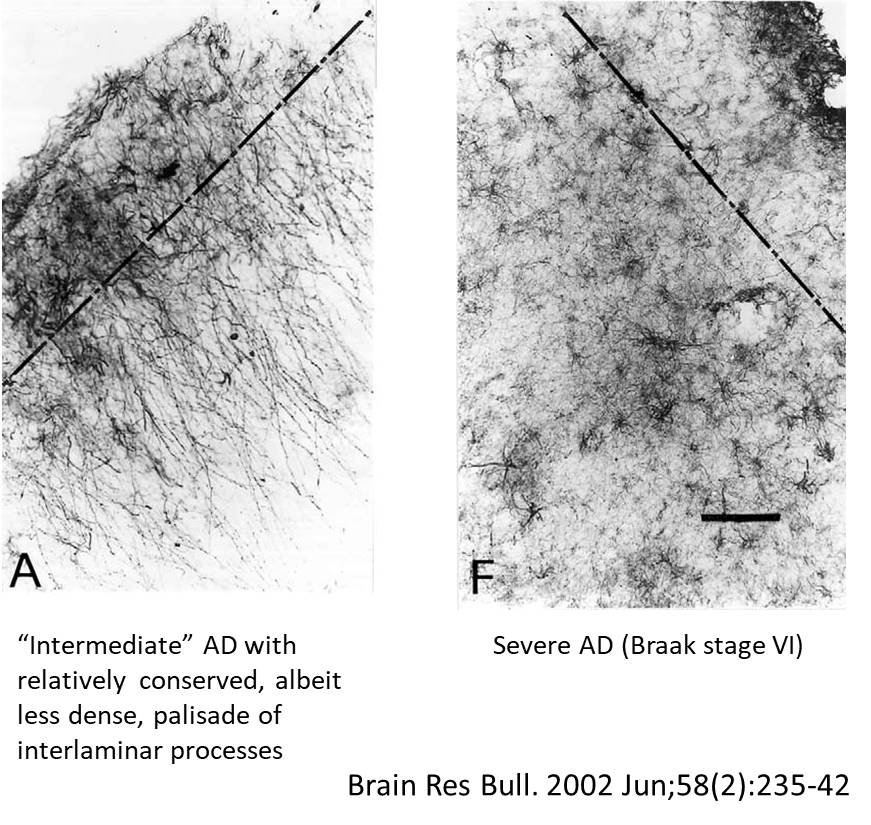
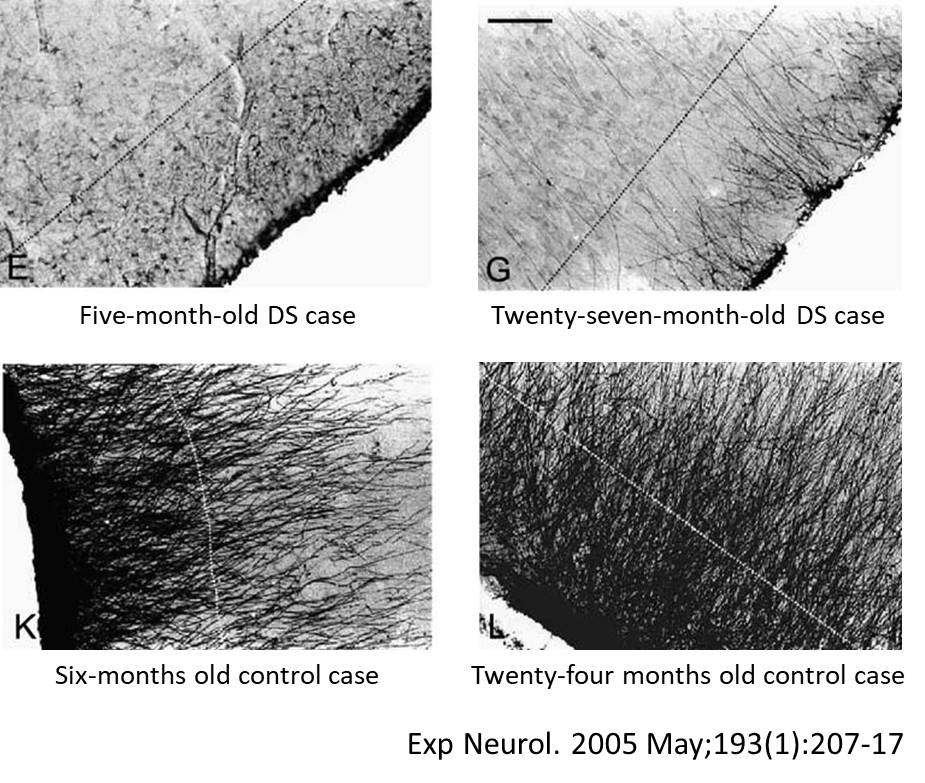
These are star-shaped cells with fine processes of variable length that differ based on their location. The major function of astrocytes is the restoration of water and ion homeostasis as well as contributing to the blood-brain barrier (BBB) maintenance (1). However, the exact role of astrocytes in the pathogenesis and progression of AD still requires thorough characterization, mainly due to a lack of human experimental data that examines the stage-dependent changes in astrocytes. Human post-mortem tissue analyses have revealed prominent reactive astrogliosis and inclusion of astrocytes into senile plaques during the late stages of AD. However, much of the understanding of their role has been based on studies with animal models. It has been observed that accumulation of Aβ could create disturbances in the Ca2+ homeostasis of astrocytes, which induce astrogliosis and cause neuroinflammation (2). Several reports have revealed that unsaturated fatty acid, e.g. palmitate, induces the NLRC4 (NLR family CARD domain-containing protein 4) expression in astrocytes and promotes AD progression (3). Interestingly, reactive astrocytes have shown to express β-secretase following occlusion of the middle cerebral artery as well as in AD mice models expressing mutant human amyloid precursor protein (APP) (4). These and several other findings have suggested that once the brain homeostasis disrupts, astrocytes can favor Alzheimer’s progression.
Ependymal cells
These cells form membrane lining of ventricles in the brain as well as the spinal cord and produce a small amount of cerebrospinal fluid, which in turn clears the accumulated Aβ from the brain during the early stages of AD (1). However, under pathological conditions, these cells have shown alteration in their lysosomal function, and instead of clearance, they promote Aβ accumulation (5). Moreover, the oxidative stress-related damage could affect the ependymal carriers (P-glycoprotein and low-density lipoprotein receptor-related protein-1) and ultimately hamper the Aβ clearance (6). In addition, these cells are also suffered from the effects of neuroinflammation, which jeopardizes the integrity of the blood-brain and the blood-CSF barrier. The pro-inflammatory molecule, nuclear factor kappa beta (NF-κB) secreted by reactive astroglia, can regulate the structural changes in ependyma, like impaired ciliary movements, which in turn affect their Aβ clearance function (7). Further, Wnt, β-catenin, and GSK-3 pathway could also alter the expression of multidrug efflux transporters and P-glycoprotein in ependymal cells, which favor the disruption of the blood-brain barrier in AD (8). A few reports have also suggested that the abundance of advanced glycation end-product receptors (RAGE) in ependymal layer could also promote the influx of glycated Aβ from blood to the brain of AD patients (9). These reports clearly show that ependymal cells play a crucial role in the clearance of Aβ from the brain.
Microglia
These are the brain macrophages, derived from the hemangioblastic mesoderm, the lineage continues through myelomonocytic cells, and finally to microglia (1). Microglia constitute 5−10% of the adult brain population (10). They are uniformly dispersed at a density of 6 mm3, and each cell occupies approximately 50,000 μm3 (11). Microglial cells express triggering receptors expressed in myeloid cells 2 (TREM2) receptors, which helps in chemotaxis, phagocytosis (besides TLR4 and CD14 receptors), migration, and proliferation. However, recent reports have suggested that in Alzheimer’s, the microglial phagocytic function has been decreased due to mutations in TREM2 (12). Microglia also express CD33, SRP-β1, and TREM1 receptors. The CD33 receptor, expressed on hematopoietic as well as immune cells, belongs to a family of sialic-acid-binding immunoglobulin-like lectins. It plays an important role in the growth of immune cells, inhibition of cytokines release from monocytes, and mediates endocytosis. Reports have shown an association between increased CD33 expression and Alzheimer’s risk, as it could inhibit the microglia-mediated Aβ uptake (13). On the other hand, signal regulatory protein β1 (SRP-β1) is a DAP-12 associated transmembrane protein, expressed on macrophages, hematopoietic cells, and microglial cells. This has a major role in the clearance of Aβ fibrils through the CD 47 ligand, which is also expressed by microglia along with other brain cells. Microglial SRP-β1 expression has found to be elevated in Alzheimer’s patients. However, in aged brains, clearance of Aβ fibrils has shown to be decreased due to a dysfunctional SRP-β1/CD 47 signaling response (14). Neuronal circuits have shown to be functionally diminished at the initial stages of AD (15). Reduction in the synapse number and inhibition of LTP leads to memory decline (16). It has been shown that in an AD brain, phagocytic functions of microglia also get reduced (17). Recently, these microglial cells have been shown to attain tolerance against continuous but low doses of LPS administration (18). Therefore, it would be interesting to check whether microglia could also become tolerant to the Aβ in AD brains or not.
Oligodendrocytes
These cells are known as the producers of myelin, which provides electrical insulation over the axons and ensures the rapid propagation of nerve impulses. Recent findings have shown that knocking out of myelin-associated genes Ugt8, Cnp, and Plp1 resulted in myelin dysfunction and eventually neurodegeneration in the mouse. These cells have also been shown to activate inflammatory pathways in response to Aβ (3). It has been found that BACE-1 plays an important role in oligodendrocyte mediated myelin production, which was confirmed by BACE inhibitor treatment (19). It has been speculated that the breakdown of myelin may promote the accumulation of toxic Aβ fibrils that ultimately form plaques in the brain (20). Moreover, Aβ has shown to induce oligodendrocyte dysfunction by activating the neutral sphingomyelinase (nSMase) – ceramide cascade via cellular anti-oxidants (21). Glutathione (GSH) precursors have shown to attenuate nSMase mediated Aβ activation and slowed down the oligodendrocyte loss, whereas GSH scavengers can enhance the nSMase activity and Aβ-induced oligodendrocyte death. Thus, oligodendrocytes can be indirectly associated with the progression of Alzheimer’s.
Conclusion
Early and reliable diagnostic markers for AD are much needed right now, therefore the time has come to look into those dark areas which have not received much attention. A better understanding of the role played by glial cells in the progression of AD during the early stages would definitely be worth a shot.
Bibliography
- Verkhratsky, A., and Butt, A. (2007) Glial Neurobiology: A Textbook, John Wiley & Sons.
- Freeman LC, Ting JP. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016 Jan;136 Suppl 1:29-38.
- Nirzhor SSR, Khan RI, Neelotpol S. The Biology of Glial Cells and Their Complex Roles in Alzheimer’s Disease: New Opportunities in Therapy. Biomolecules. 2018 Sep 10;8(3).
- Rossner S, Lange-Dohna C, Zeitschel U, Perez-Polo JR. Alzheimer’s disease beta-secretase BACE1 is not a neuron-specific enzyme. J Neurochem. 2005 Jan;92(2):226-34.
- Balusu S, Brkic M, Libert C, Vandenbroucke RE. The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: more than just a barrier. Neural Regen Res. 2016 Apr;11(4):534-7.
- van Assema DM, Lubberink M, Bauer M, van der Flier WM, Schuit RC, Windhorst AD, et al. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain. 2012 Jan;135(Pt 1):181-9.
- Lattke M, Magnutzki A, Walther P, Wirth T, Baumann B. Nuclear factor kappaB activation impairs ependymal ciliogenesis and links neuroinflammation to hydrocephalus formation. J Neurosci. 2012 Aug 22;32(34):11511-23.
- Liu L, Wan W, Xia S, Kalionis B, Li Y. Dysfunctional Wnt/beta-catenin signaling contributes to blood-brain barrier breakdown in Alzheimer’s disease. Neurochem Int. 2014 Sep;75:19-25.
- Maslinska D, Laure-Kamionowska M, Taraszewska A, Deregowski K, Maslinski S. Immunodistribution of amyloid beta protein (Abeta) and advanced glycation end-product receptors (RAGE) in choroid plexus and ependyma of resuscitated patients. Folia Neuropathol. 2011;49(4):295-300.
- Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev Neurosci. 2011;33(3-4):199-209.
- Lee CY, Landreth GE. The role of microglia in amyloid clearance from the AD brain. J Neural Transm (Vienna). 2010 Aug;117(8):949-60.
- Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol. 2018 Feb 5;217(2):459-72.
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013 May 22;78(4):631-43.
- Floden AM, Combs CK. Microglia demonstrate age-dependent interaction with amyloid-beta fibrils. J Alzheimers Dis. 2011;25(2):279-93.
- Zott B, Busche MA, Sperling RA, Konnerth A. What Happens with the Circuit in Alzheimer’s Disease in Mice and Humans? Annu Rev Neurosci. 2018 Jul 8;41:277-97.
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016 Jun;8(6):595-608.
- Spittau B. Aging Microglia-Phenotypes, Functions and Implications for Age-Related Neurodegenerative Diseases. Front Aging Neurosci. 2017;9:194.
- Lelios I, Greter M. Trained Microglia Trigger Memory Loss. Immunity. 2018 May 15;48(5):849-51.
- McKenzie AT, Moyon S, Wang M, Katsyv I, Song WM, Zhou X, et al. Multiscale network modeling of oligodendrocytes reveals molecular components of myelin dysregulation in Alzheimer’s disease. Mol Neurodegener. 2017 Nov 6;12(1):82.
- Bartzokis G, Lu PH, Mintz J. Human brain myelination and amyloid beta deposition in Alzheimer’s disease. Alzheimers Dement. 2007 Apr;3(2):122-5.
- Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, et al. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 2004 Jan 5;164(1):123-31.